You get access to a software in which structured, standardized reports for diagnostic and research purposes can be created by means of digital forms.
We provide you with a variety of multilingual, intelligent forms for pathological (cancer & non-cancer) and cytopathological reporting. The forms we have developed comply with current international standards and are updated on an ongoing basis.
Our software allows you to customize forms according to your needs by hiding optional groups, renaming elements, and pre-filling values. In addition to the default selection options, all forms also contain a number of (mostly optional) free-text fields.
Statistical exports can be created directly from the data pool and first analyses can be performed immediately in the integrated statistics tool.
Data can be easily transported to and from surrounding systems. Supported data formats: Json/Xml/Soap/Rest/File-based/.DB).
The software is highly available and is already in productive use at several hospitals. Software support is included in the license costs.
Included in the package of services we offer:
-SynReport, a tool for structured reporting in pathology (cancer/non-cancer, including cytopathology). Our software features highly-adaptive, touchscreen-compatible user interfaces and is capable of integrating information directly from electronic orders and analytical devices:
-A variety of intelligent web-based forms that guide the pathologist along organ-specific decision trees through the creation of a report. We provide ready-to-use forms that are created based on templates from the International Collaboration on Cancer Reporting (ICCR), but forms can also be customized if needed: form elements can be added or hidden, and ready-made text modules can be modified. The forms are provided in multiple languages and are regularly adapted to the latest standards. We are continuously developing additional forms based on the published ICCR templates, and are also happy to produce customer-specific forms on request.
A statistics tool for downloading the data generated in the background, in which initial statistical evaluations can also be performed. All data resulting from the use of the report forms belong to the customer. Celerato concludes contracts according to the general terms and conditions of the Swiss IT Conference.
Our software generates formatted medical reports in text form, including the appropriate medical codes (WHO ICD-O 3.2, SNOMED CT, UICC TNM) based on the selected elements, as well as raw data and filterable statistical data for research purposes and further analysis using artificial intelligence (AI).
Hardware requirements: 2 CPUs, 8GB RAM
Current browser: Chrome, Firefox, Edge
If you choose the cloud solution, this meets all the requirements and you can get started right away. If you prefer to install on-site (on-premises), a server with installed operating system (Windows or Linux), a SQL database (SQLServer or PostgreSQL) and the possibility of remote access for support purposes are also required.
-Time and cost savings: creating a report using our forms is significantly faster and less expensive than creating it in the traditional way (dictation and transcription). In addition, because we update the forms on an ongoing basis (see next item), clinicians who use our service no longer necessarily have to be concerned with the constantly changing standards. Last but not least, the instructional nature of our solution also makes it much less time-consuming to train prospective doctors.
-Improved report quality: our forms are regularly adapted to the latest versions of the ICCR form templates, as well as to the latest edition of the UICC TNM, so that the reports always comply with the current standards at the time of creation. In addition, automatic text generation and import of analysis data helps eliminate clerical errors and misunderstandings.
-Improved data quality: reports generated using our forms contain easily accessible, standardized data. This greatly simplifies the statistical analysis of medical data, which of course has enormous potential, especially for research purposes. The codes generated in the background can also be automatically sent to other data processors (e.g. HIS, cancer registries) on a standard basis.
SynReport is a tool for structured reporting of level 6. These stages/levels are described and visualized in detail in the image "Structured Reporting - Levels of Implementation". To highlight the differences, the defining properties of levels 1-6 are also listed below (the features of the previous levels are included in each of the subsequent levels).
Level 1: Reports in narrative language.
Level 2: Reports with locally standardized content.
Level 3: Reports that are structured according to specific criteria.
Level 4: Reports are created electronically (web form) with completeness check.
Level 5: Reports with internationally standardized content.
Level 6: Reports in which important form elements reflect internationally recognized codes.
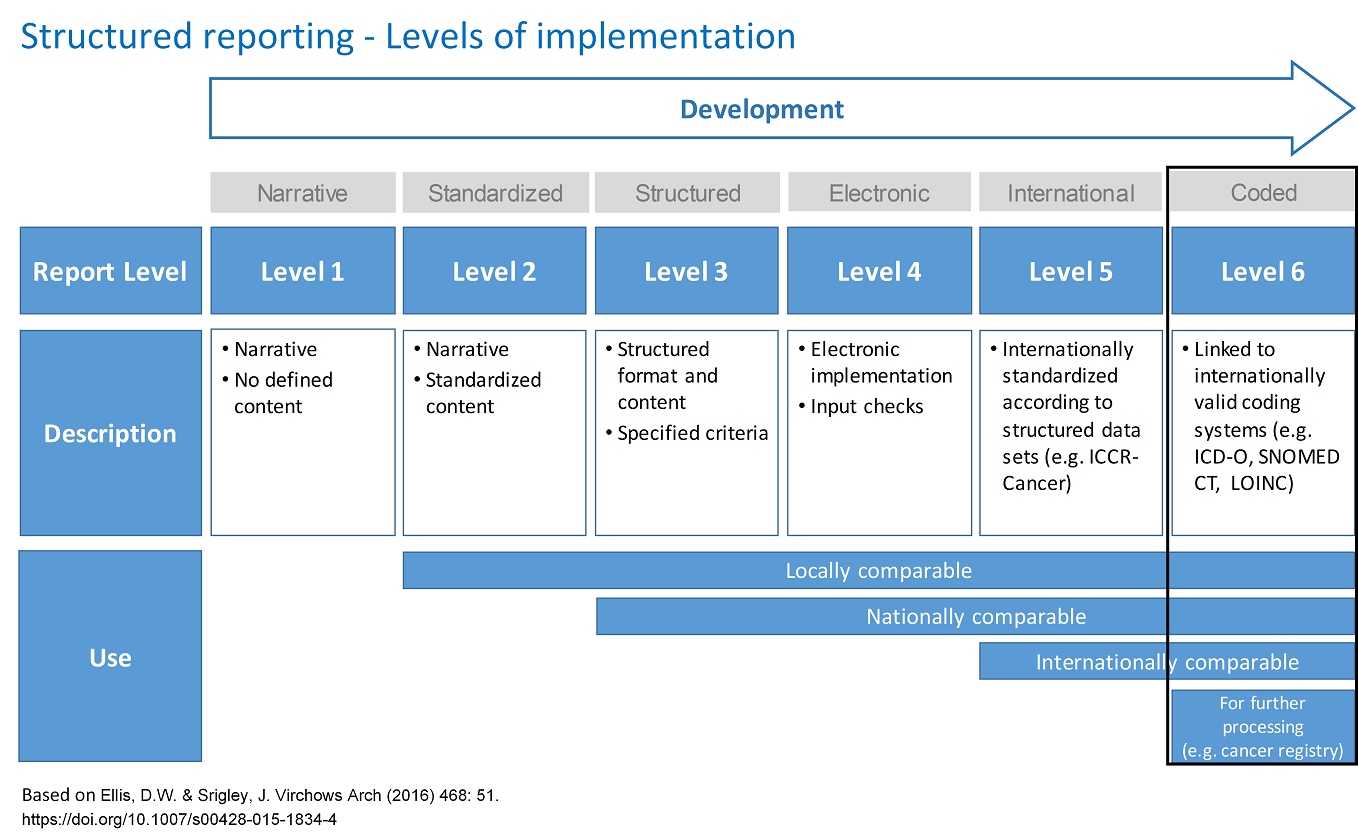
Legend:
ICCR-Cancer: International Collaboration on Cancer Reporting
.
ICD-O: International Classification of Diseases for Oncology
SNOMED CT: Systematized Nomenclature of Medicine - Clinical Terms
LOINC: Logical Observation Identifiers Names and Codes
As part of the "PathoLink" project funded by the Swiss National Science Foundation (SNSF), a software for structured reporting is being developed under the name "SynReport". This software is initially being tested at four Swiss pathology institutes.
As a result of the positive feedback from the participating institutes, as well as expressions of interest from institutes external to Patholink, the decision is made to found a company to further develop the software.
The registration in the commercial register and the transfer of the rights to SynReport to Celerato ensue.
Software and reporting forms are being further developed and SynReport is being implemented in the large pathology institutes in Bern, Basel and Zurich. In addition, Celerato is able to acquire its first customer in Germany.
Celerato strengthens its collaboration with the International Collaboration on Cancer Reporting (ICCR) through a finalised Memorandum of Understanding (MoU) and gains access to internationally recognised cancer protocols.
Celerato participates in a software trial organised by the Royal College of Pathologists of Australasia (RCPA). Click here to learn more about our participation in this trial embedded in the Structured Pathology Reporting of Cancer (SPRC) Project.

CEO & CTO

Quality Management & Form Development

Sales & Administration
Software Development